Length: SVL: 200-280mm, with the tail being equal to or slightly longer than the body length
Weight: Females up to 0.5kg; Males up to 1kg
Tuatara are the sole surviving representatives of the order Rhynchocephalia. Despite a close resemblance, tuatara are not lizards. Informally known as "living fossils", they are thought to have remained essentially unchanged for 150 million years. The tuatara is New Zealand's largest endemic reptile, reaching 200-280mm SVL (snout-vent-length). Until recently, two species of tuatara were recognised: Sphenodon punctatus (Northern tuatara) and Sphenodon guntheri (Brother's Island tuatara), however, taxonomic revision has recently synonymised them into a single species:S. punctatus.
Description
Tuatara are generally olive green to slate grey in colour with a fine speckling across the body, though some individuals (particularly in the northern part of their range) may have red, orange, or yellowish brown tones. Tuatara have relatively soft skin and undergo ecdysis (loss of skin) at least once a year, allowing the colouration to change over the animals lifetime. Tuatara are sexually dimorphic, with males being larger than females. A crest of spines runs along the length of the back and tail (tuatara means ‘peaks on back’ in Maori), and the crest is more pronounced in males.
Adults have a ridge running from each eye to the snout and no external ear hole. Tuatara have a parietal or ‘third’ eye which is photoreceptive and aids in setting circadian and seasonal cycles. Tuatara have a unique tooth arrangement with the upper jaw having two rows of teeth, while the lower jaw has only one row which slides between the two upper rows. The teeth are actually serrated protrusions of the jaw bone. Tuatara are the most unspecialised amniote in the world with the most primitive heart of any reptile. The lungs have only one chamber with no bronchi (branches or tracts within the lung).
Life expectancy
The lifespan of tuatara probably exceeds 100 years.
Distribution
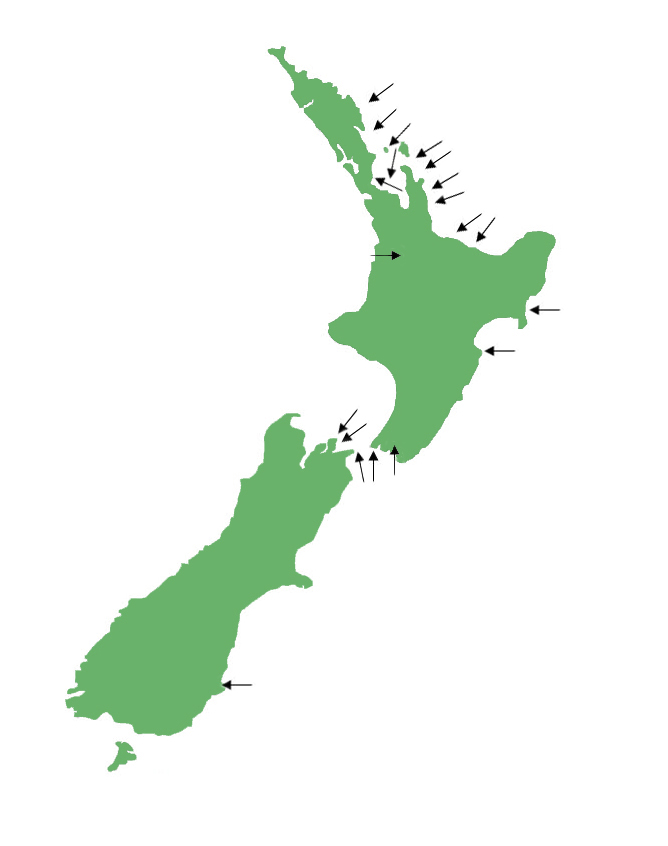
Tuatara are present on offshore islands in the Cook Straight and on the east coast of the North Island. In recent years tuatara have been introduced into predator proof ‘mainland islands’ such as Zealandia sanctuary in Wellington. There are very few places where you can see tuatara in the wild with the majority of the populations residing on protected offshore islands, off limits to the public. To see tuatara, visit one of the many captive facilities we list on the website, or one of the publicly accessible sanctuaries (e.g. Tiritiri Matangi Island, Motuihe Island, Matiu/Somes Island, Zealandia, Orokonui) where you may be lucky enough to see one sunbasking alongside a track!
Ecology and habitat
Adult tuatara are nocturnal (though they will sun bask), while young are diurnal; an evolutionary development which is thought to have arisen because adult tuatara will cannibalise young. The species are able to remain acitve at temperatures as low as 5°C, but will commonly hibernate during the winter. Tuatara inhabit coastal forest and clearings, using burrows for shelter (either sequestering bird burrows or digging their own), sharing habitat with sea birds such as shearwaters and petrels.
Social structure
Tuatara are solitary, however, a number of tuatara will share a burrow system. Both sexes will defend their tunnel segment and territory fiercely. Males will make croaking sounds during confrontations.
Breeding biology
The reproductive rate of tuatara is very slow with females laying a clutch of eggs every 4 years. Mating takes place in midsummer; during courtship the male will raise his crest, deepen his colour and parade for the female. Gestation is 11 – 16 months, after hatching tuatara take around 13 years to reach full maturity. Tuatara have temperature dependent sex determination with more males produced when eggs are incubated at a warmer temperature, while more females are produced when incubation occurs under cooler conditions.
Diet
Three quarters of their diet is invertebrates, and the remainder consists of smaller lizards, birds, and other organic material. Tuatara teeth are not replaced throughout the lifetime; elderly tuatara often switch to softer prey as their teeth wear down.
Disease
Ticks can infest tuatara and are difficult to see as they blend with the colour of skin and scale pattern. The nematode Hatterianema hollandiae and several types of protozoa have also been found in tuatara. Known host for the blood paraite Hepatozoon tuatarae.
They are known hosts for the trematode Dolichosaccus leiolopismae, a species seemingly restricted to Aotearoa's reptile fauna (Allison & Blair, 1987).
Conservation strategy
DOC classify tuatara as 'uncommon' with more than 20,000 mature individuals, a stable population or increasing by more than 10%.
Click here for a copy of the species RECOVERY PLAN.
Click here to learn about CAPTIVE MANAGEMENT AND HUSBANDRY of tuatara.
References
Ainsworth, R. (1994). Parasites of NZ reptiles, poster paper for the Second World Congress of Herpetology, Adelaide.
Alibardi, L., & Meyer-Rochow, V. B. (2021). Regeneration in Reptiles Generally and the New Zealand Tuatara in Particular as a Model to Analyse Organ Regrowth in Amniotes: A Review. Journal of Developmental Biology, 9(3), 36.
Allison, B., & Blair, D. (1987). The genus Dolichosaccus (Platyhelminthes: Digenea) from amphibians and reptiles in New Zealand, with a description of Dolichosaccus (Lecithopyge) leiolopismae n. sp. New Zealand journal of zoology, 14(3), 367-374.
Apesteguía, S., Garberoglio, F. F., & Gómez, R. O. (2021). Earliest tuatara relative (Lepidosauria: Sphenodontinae) from southern continents. Ameghiniana, 58(5), 416-441.
Apesteguía, S., & Jones, M. E. H. (2012). A late cretaceous “tuatara”(Lepidosauria: Sphenodontinae) from South America. Cretaceous Research, 34, 154-160.
Browne, C. M., Stafford, K. J., & Fordham, R. A. (2015). The detection and identification of tuatara and gecko scents by dogs. Journal of Veterinary Behavior, 10(6), 496-503.
Buller, W.L. (1879). Notes on the tuatara lizard (Sphenodon) from East Cape Island. Proceedings of the New Zealand Institute, 1877, 10, 220-221.
Castanet, J., Newman, D. G., & Girons, H. S. (1988). Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica, 25-37.
Cree, A., Cockrem, J. F., & Guillette, L. J. (1992). Reproductive cycles of male and female tuatara (Sphenodon punctatus) on Stephens Island, New Zealand. Journal of Zoology, 226(2), 199-217.
Daugherty, C. H., Cree, A., Hay, J. M., & Thompson, M. B. (1990). Neglected taxonomy and continuing extinctions of tuatara (Sphenodon). Nature, 347(6289), 177-179.
East, K. T., East, M. R., & Daugherty, C. H. (1995). Ecological restoration and habitat relationships of reptiles on Stephens Island, New Zealand. New Zealand Journal of Zoology, 22(3), 249-261.
Feldman, A., Sabath, N., Pyron, R. A., Mayrose, I., & Meiri, S. (2016). Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Global Ecology and Biogeography, 25(2), 187-197.
Fund, K., Fund, B., Plans, S., Taiao, T. T., Grants, S. T., & Scheme, N. R. M. (1981). Feeding ecology of the tuatara (Sphenodon punctatus) on Stephens Island, Cook Strait. New Zealand journal of ecology, 4, 89-97.
Gartrell, B. D., Youl, J. M., King, C. M., Bolotovski, I., McDonald, W. L., & Nelson, N. J. (2007). Failure to detect Salmonella species in a population of wild tuatara (Sphenodon punctatus). New Zealand veterinary journal, 55(3), 134-136.
Gaze, P. (2001). Tuatara recovery plan 2001–2011: Threatened species recovery plan 47. Wellington: Department of Conservation.
Gemmell, N. J., Rutherford, K., Prost, S., Tollis, M., Winter, D., Macey, J. R., Adelson. D. L., Suh, A., Bertozzi, T., Grau, J. H., Organ, C., Gardner, P. P., Muffato, M., Patricio, M., Billis, K., Martin, F. J., Flicek, P., Petersen, B., Kang, L., Michalak, P., Buckley, T. R., Wilson, M., Cheng, Y., Miller, H., Schott, R. K., Jordan, M. D., Newcomb, R. D., Arroyo, J. I., Valenzuela, N., Hore, T. A., Renart, J., Peona, V., Peart, C. R., Warmuth, V. M., Zeng, L., Kortschak, R. D., Raison, J. M., Zapata, V. V., Wu, Z., Santesmasses, D., Mariotti, M., Guigó, R., Rupp, S. M., Twort, V. G., Dussex, N., Taylor, H., Abe, H., Bond, D. M., Paterson, J. M., Mulcahy, D. G., Gonzalez, V. L., Barbieri, C. G., DeMeo, D. P., Pabinger, S., Van Stijn, T., Clarke, S., Ryder, O., Edwards, S. V., Salzberg, S. L., Anderson, L., Nelson, N., Stone, C., & Ngatiwai Trust Board (2020). The tuatara genome reveals ancient features of amniote evolution. Nature, 584(7821), 403-409.
Gill, B., & Whitaker, T. (2007). New Zealand frogs and reptiles. Auckland: David Bateman Limited.
Gillingham, J. C., Carmichael, C., & Miller, T. (1995). Social behavior of the tuatara, Sphenodon punctatus. Herpetological monographs, 5-16.
Godfrey, S. S., Moore, J. A., Nelson, N. J., & Bull, C. M. (2010). Social network structure and parasite infection patterns in a territorial reptile, the tuatara (Sphenodon punctatus). International journal for parasitology, 40(13), 1575-1585.
Gorniak, G. C., Rosenberg, H. I., & Gans, C. (1982). Mastication in the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): structure and activity of the motor system. Journal of Morphology, 171(3), 321-353.
Grayson, K. L., Mitchell, N. J., Monks, J. M., Keall, S. N., Wilson, J. N., & Nelson, N. J. (2014). Sex ratio bias and extinction risk in an isolated population of tuatara (Sphenodon punctatus). PloS one, 9(4), e94214.
Hay, J. M., Sarre, S. D., Lambert, D. M., Allendorf, F. W., & Daugherty, C. H. (2010). Genetic diversity and taxonomy: a reassessment of species designation in tuatara (Sphenodon: Reptilia). Conservation Genetics, 11(3), 1063-1081.
Herrel, A., Moore, J. A., Bredeweg, E. M., & Nelson, N. J. (2010). Sexual dimorphism, body size, bite force and male mating success in tuatara. Biological Journal of the Linnean Society, 100(2), 287-292.
Herrera‐Flores, J. A., Stubbs, T. L., & Benton, M. J. (2017). Macroevolutionary patterns in Rhynchocephalia: is the tuatara (Sphenodon punctatus) a living fossil?. Palaeontology, 60(3), 319-328.
Hitchmough, R., Barr, B., Knox, C., Lettink, M., Monks, J. M., Patterson, G. B., Reardon, J. T., van Winkel, D., Rolfe, J., & Michel, P. (2021). Conservation status of New Zealand reptiles, 2021. New Zealand threat classification series 35. Wellington: New Zealand Department of Conversation.
Hitchmough, R.A., Barr, B., Lettink, M., Monks, J., Reardon, J., Tocher, M., van Winkel, D., Rolfe, J. (2016). Conservation status of New Zealand reptiles, 2015; New Zealand threat classification series 17. Wellington: New Zealand Department of Conservation.
Holthaus, K. B., Alibardi, L., Tschachler, E., & Eckhart, L. (2020). Identification of epidermal differentiation genes of the tuatara provides insights into the early evolution of lepidosaurian skin. Scientific reports, 10(1), 1-11.
Howes, G. B., & Swinnerton, H. H. (1901). On the development of the skeleton of the tuatara, Sphenodon punctatus; with remarks on the egg, on the hatching, and on the hatched young. The Transactions of the Zoological Society of London, 16(1), 1-84.
Jarvie, S., Besson, A. A., Seddon, P. J., & Cree, A. (2014). Assessing thermal suitability of translocation release sites for egg‐laying reptiles with temperature‐dependent sex determination: a case study with tuatara. Animal Conservation, 17, 48-55.
Jones, M. E., Anderson, C. L., Hipsley, C. A., Müller, J., Evans, S. E., & Schoch, R. R. (2013). Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC evolutionary biology, 13(1), 1-21.
Jewell, T. (2011). A photographic guide to the reptiles and amphibians of New Zealand. Auckland: New Holland Publishers.
Jones, M. E., Tennyson, A. J., Worthy, J. P., Evans, S. E., & Worthy, T. H. (2009). A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon). Proceedings of the Royal Society B: Biological Sciences, 276(1660), 1385-1390.
Laird, M. (1950). Haemogregarina tuatarae sp. n., from the New Zealand Rhynchocephalian Sphenodon punctatus (Gray). Proceedings of the Zoological Society of London, 120(3), 529-533.
McCallum, J. (1980). Reptiles of the northern Mokohinau Group. Tane, 26, 53-59.
McCallum, J. (1981). Reptiles of Tawhiti Rahi Island, Poor Knights Islands, New Zealand. Tane, 27, 55-58.
McCallum, J. (1981). Reptiles of the North Cape region, New Zealand. Tane, 27, 152-157.
McCallum, J., & Harker, F. R. (1981). Reptiles of Cuvier Island. Tane, 27, 17-22.
McCallum, J., & Harker, F. R. (1982). Reptiles of Little Barrier Island. Tane, 28, 21-27.
Melzer, S., Hitchmough, R., van Winkel, D., Wedding, C., Chapman, S., & Rixon, M. (2022). Conservation Status of Reptile Species in Tāmaki Makaurau/Auckland. Auckland Council technical report TR2022/3.
Middleton, D. M., La Flamme, A. C., Gartrell, B. D., & Nelson, N. J. (2014). Reptile reservoirs and seasonal variation in the environmental presence of Salmonella in an island ecosystem, Stephens Island, New Zealand. Journal of wildlife diseases, 50(3), 655-659.
Miller, H. C., Allendorf, F. W., & Daugherty, C. H. (2010). Genetic diversity and differentiation at MHC genes in island populations of tuatara (Sphenodon spp.). Molecular Ecology, 19(18), 3894-3908.
Miller, K. A., Miller, H. C., Moore, J. A., Mitchell, N. J., Cree, A., Allendorf, F. W., Sarre, S. D., Keall, S. N., & Nelson, N. J. (2012). Securing the demographic and genetic future of tuatara through assisted colonization. Conservation Biology, 26(5), 790-798.
Miller, H. C., Moore, J. A., Allendorf, F. W., & Daugherty, C. H. (2009). The evolutionary rate of tuatara revisited. Trends in Genetics, 25(1), 13-15.
Mitchell, N. J., Allendorf, F. W., Keall, S. N., Daugherty, C. H., & Nelson, N. J. (2010). Demographic effects of temperature‐dependent sex determination: will tuatara survive global warming?. Global Change Biology, 16(1), 60-72.
Mitchell, N. J., Kearney, M. R., Nelson, N. J., & Porter, W. P. (2008). Predicting the fate of a living fossil: how will global warming affect sex determination and hatching phenology in tuatara?. Proceedings of the Royal Society B: Biological Sciences, 275(1648), 2185-2193.
Moore, J. A., Daugherty, C. H., & Nelson, N. J. (2009). Large male advantage: phenotypic and genetic correlates of territoriality in tuatara. Journal of Herpetology, 43(4), 570-578.
Nelson, N. J., Keall, S. N., Brown, D., & Daugherty, C. H. (2002). Establishing a new wild population of tuatara (Sphenodon guntheri). Conservation Biology, 16(4), 887-894.
Nelson, N. J., Keall, S. N., Pledger, S., & Daugherty, C. H. (2002). Male‐biased sex ratio in a small tuatara population. Journal of Biogeography, 29(5‐6), 633-640.
Ramstad, K. M., Nelson, N. J., Paine, G., Beech, D., Paul, A., Paul, P., Allendorf, F. W., & Daugherty, C. H. (2007). Species and cultural conservation in New Zealand: Maori traditional ecological knowledge of tuatara. Conservation Biology, 21(2), 455-464.
Rest, J. S., Ast, J. C., Austin, C. C., Waddell, P. J., Tibbetts, E. A., Hay, J. M., & Mindell, D. P. (2003). Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome. Molecular phylogenetics and evolution, 29(2), 289-297.
Scharf, I., Feldman, A., Novosolov, M., Pincheira‐Donoso, D., Das, I., Böhm, M., Uetz, P., Torres-Carvajal, O., Bauer, A., Roll, U., & Meiri, S. (2015). Late bloomers and baby boomers: ecological drivers of longevity in squamates and the tuatara. Global Ecology and Biogeography, 24(4), 396-405.
Subramanian, S., Hay, J. M., Mohandesan, E., Millar, C. D., & Lambert, D. M. (2009). Molecular and morphological evolution in tuatara are decoupled. Trends in Genetics, 25(1), 16-18.
Thompson, M. B., Daugherty, C. H., Cree, A., French, D. C., Gillingham, J. C., & Barwick, R. E. (1992). Status and longevity of the tuatara, Sphenodon guntheri, and Duvaucel's gecko, Hoplodactylus duvaucelii, on North Brother Island, New Zealand. Journal of the Royal Society of New Zealand, 22(2), 123-130.
Thompson, M. B., Packard, G. C., Packard, M. J., & Rose, B. (1996). Analysis of the nest environment of tuatara Sphenodon punctatus. Journal of Zoology, 238(2), 239-251.
Towns, D. R. (1972). The reptiles of Red Mercury Island. Tane, 18, 95-105.
Towns, D. R. (1991). Response of lizard assemblages in the Mercury Islands, New Zealand, to removal of an introduced rodent: the kiore (Rattus exulans). Journal of the Royal Society of New Zealand, 21(2), 119-136.
Towns, D. R., & Hayward, B. W. (1973). Reptiles of the Aldermen Islands. Tane, 19, 93-102.
Tyrrell, C. L., & Cree, A. (1998). Relationships between corticosterone concentration and season, time of day and confinement in a wild reptile (tuatara, Sphenodon punctatus). General and comparative endocrinology, 110(2), 97-108.
Walls, G. Y. (1983). Activity of the tuatara and its relationships to weather conditions on Stephens Island, Cook Strait, with observations on geckos and invertebrates. New Zealand journal of zoology, 10(3), 309-317.
Webster, R. K. E. (2018). The epidemiology and pathology of Paranannizziopsis australasiensis in New Zealand reptiles: a thesis presented in partial fulfilment of the requirements for the degree of Master of Veterinary Science in Wildlife Health at Massey University, Palmerston North, New Zealand (Doctoral dissertation, Massey University).
Werner, Y. L., & Whitaker, A. H. (1978). Observations and comments on the body temperatures of some New Zealand reptiles. New Zealand Journal of Zoology, 5(2), 375-393.
Wilson, J. (2010). Population viability and resource competition on North Brother Island: conservation implications for tuatara (Sphenodon punctatus) and Duvaucel's gecko (Hoplodactylus duvaucelii).A thesis submitted as partial fulfilment of the requirements for the degree of Master of Science in Conservation Biology. Victoria University of Wellington.
Worthy, T. H. (1998). Quaternary fossil faunas of Otago, South Island, New Zealand. Journal of the Royal Society of New Zealand, 28(3), 421-521.
Worthy, T. H., & Holdaway, R. N. (1995). Quaternary fossil faunas from caves on Mt Cookson, North Canterbury, South Island, New Zealand. Journal of the Royal Society of New Zealand, 25(3), 333-370.